Photo Courtesy Of MassDevice.com
- 3rd FDA panel vote barely favors Boston Scientific’s Watchman anti-stroke device
- Medtronic launches Verify incontinence device in the U.S
- FDA OKs Integra Lifesciences Puerto Rico plant; FDA approves Bard’s Lutonix DEB
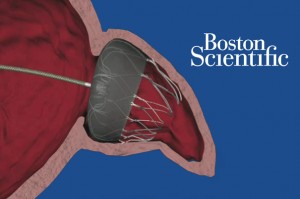
An FDA advisory panel offered a mixed view of Boston Scientific’s Watchman heart device earlier this month, concluding it is probably safe but not particularly effective in reducing the risk of stroke in patients with a certain type of irregular heart beat.
Despite the lack of likely effectiveness, the panel voted 6-5, with 1 abstention, that the benefits outweigh the risks, with some panelists saying it should be an option for patients.
[button link=”http://www.massdevice.com/news/update-3rd-fda-panel-vote-barely-favors-boston-scientifics-watchman-anti-stroke-device” color=”silver” window=”yes”]Click Here To Read More[/button]
Source: www.massdevice.com; October 13, 2014.







